Master Lewis Structures: Step-by-Step Drawing Guide

Mastering Lewis structures is essential for anyone studying chemistry, as they provide a visual representation of the distribution of electrons in a molecule. Whether you're a student preparing for exams or a professional needing a refresher, this step-by-step guide will help you draw Lewis structures with confidence. By following these instructions, you’ll learn to accurately depict molecular bonding and lone pairs, ensuring a deeper understanding of chemical compounds. (Lewis structures, chemical bonding, molecular geometry)
Step 1: Count Total Valence Electrons

The first step in drawing a Lewis structure is determining the total number of valence electrons in the molecule. Valence electrons are the electrons in the outermost shell of an atom, which participate in bonding. Follow these steps:
- Identify the elements in the molecule.
- Look up their atomic numbers to find the number of valence electrons.
- Sum the valence electrons for all atoms in the molecule.
📌 Note: For ions, add or subtract electrons based on the charge.
Step 2: Determine the Central Atom

Next, identify the central atom in the molecule. This is usually the least electronegative atom or the one that can form the most bonds. For example, in water (H₂O), oxygen is the central atom. (Central atom, molecular structure)
Step 3: Connect Atoms with Single Bonds

Place the central atom in the middle and connect the surrounding atoms with single bonds. Each single bond represents two electrons. This step helps you visualize the basic framework of the molecule. (Single bonds, molecular framework)
Step 4: Distribute Remaining Electrons
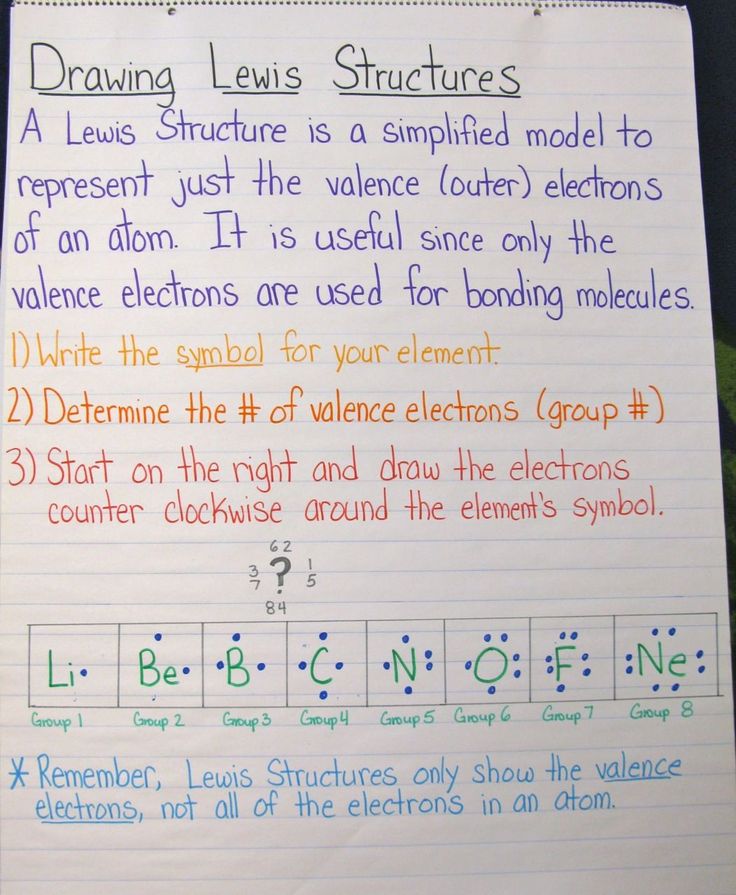
After forming the bonds, distribute the remaining valence electrons as lone pairs on the atoms, starting with the outer atoms. Ensure each atom (except hydrogen) follows the octet rule, which states that atoms aim to have eight electrons in their outer shell. (Octet rule, electron distribution)
Step 5: Check for Formal Charges

Calculate the formal charge for each atom to ensure the structure is stable. The formal charge is calculated as: Formal Charge = Valence Electrons – Lone Pair Electrons – (Bonding Electrons / 2). Aim for a structure with the lowest formal charges. (Formal charge, stable structure)
Step 6: Adjust for Exceptions
Some molecules do not follow the octet rule, such as those with an expanded octet or odd-electron molecules. Adjust your structure accordingly to reflect these exceptions. (Expanded octet, odd-electron molecules)
Checklist for Drawing Lewis Structures
- Count total valence electrons.
- Identify the central atom.
- Connect atoms with single bonds.
- Distribute remaining electrons as lone pairs.
- Check formal charges for stability.
- Adjust for exceptions to the octet rule.
By following this step-by-step guide, you’ll be able to master Lewis structures and tackle any molecular diagram with ease. Practice regularly to reinforce your skills and deepen your understanding of chemical bonding. (Lewis structures, chemical bonding, molecular geometry)
What is the octet rule in Lewis structures?
+
The octet rule states that atoms tend to gain, lose, or share electrons to achieve a full outer shell of eight electrons, except for hydrogen, which needs only two.
How do I calculate formal charge?
+
Formal charge is calculated as: Valence Electrons – Lone Pair Electrons – (Bonding Electrons / 2). It helps determine the most stable Lewis structure.
What are exceptions to the octet rule?
+
Exceptions include atoms with an expanded octet (like sulfur or phosphorus) and odd-electron molecules (like nitric oxide, NO).

